 |
15106-1 Humidity detection sensor method Links to the methods show one of possibly several examples of implementation. These links are merely examples for basic information and do not constitute any kind of technical opinion or endorsement. |
ISO Standards
 |
15106-1 Humidity detection sensor method Links to the methods show one of possibly several examples of implementation. These links are merely examples for basic information and do not constitute any kind of technical opinion or endorsement. |
Other Methods
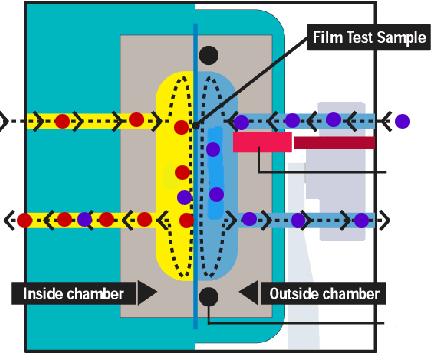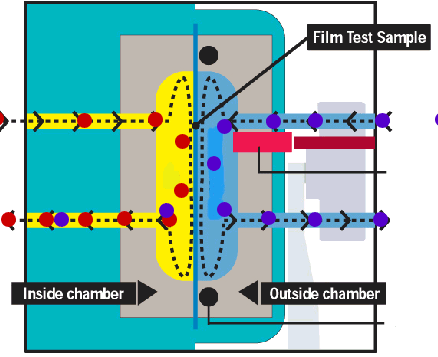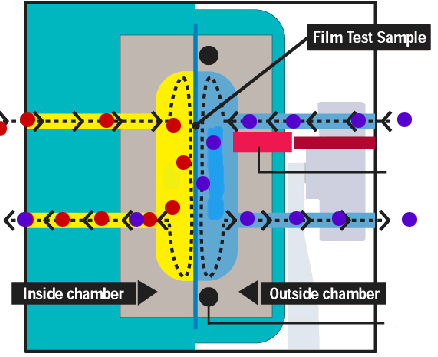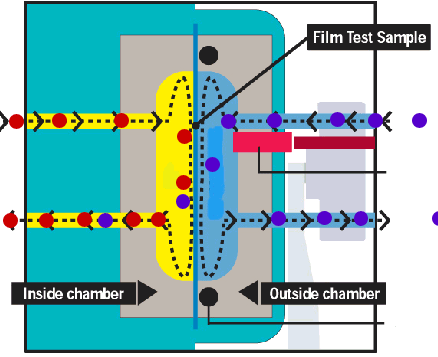 |
Mocon sensor The feeding side of the membrane is exposed to the gas or vapour to be studied, this can be done statically or with a continuous stream of permeant gas to keep a constant pressure. On the permeant side the permeated gas or vapour is swept away with a carrier gas (usually nitrogen) and fed into a sensor. Rather than measuring some absolute quantity of the mixture permeant+carrier gas a relative difference between the mixture permeant+carrier gas and the pure carrier gas is frequently used. In principle, these type of methods gives continuous readings. Example www.mocon.com |
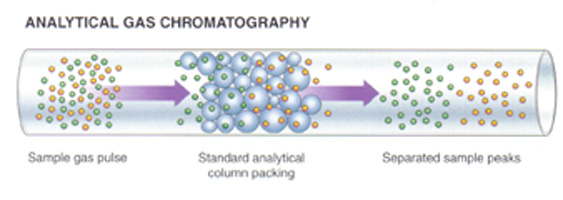 |
Gas chromatograph The gas chromatograph is a detector that can distinguish between species of different mass, because they travel at
different velocities. The gas chromatograph can also be used as detector after gas or vaour has accumulated in a vessel after permeation. |
|
Thermistors Depending on an amount of gas present around a thermistor the characteristics of the thermistor changes.
This is similar to the principle used in pirani gauges for pressure measurements.
By using a Wheatstone bridge with one thermistor in the pure carrier gas and one
thermistor in the permeant+carrier gas the accuracy of the method can be increased. |
|
Infrared sensors Infrared sensors can be used to detect suitable permeants such as water vapour
by detecting changes in the IR-absorption spectrum. For more details on development
of instruments for WVTR measurement see: |
|
Chemical sensors There are a variety of chemical sensors, which are in many cases specific to a certain permeant. The picture shows a ZrO2 sensor for oxygen detection. One side of the sensor is kept at a constant oxygen partial pressure. The Nernst equation relates the unknown oxygen concentration to the voltage measured accross the electrodes. V=RT/4F*ln(pO2WE/pO2RE) |
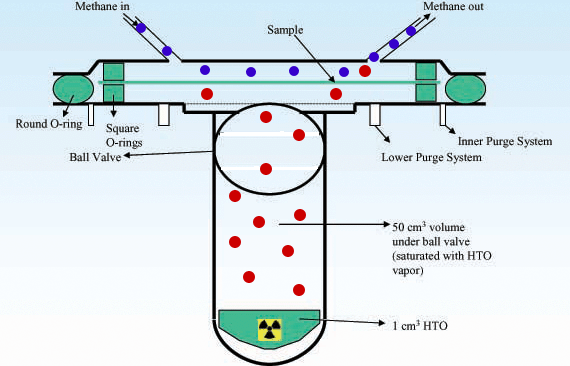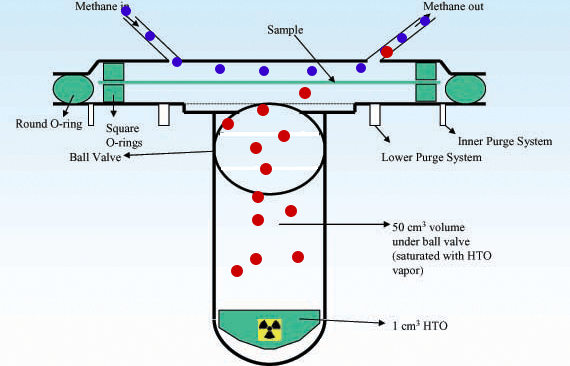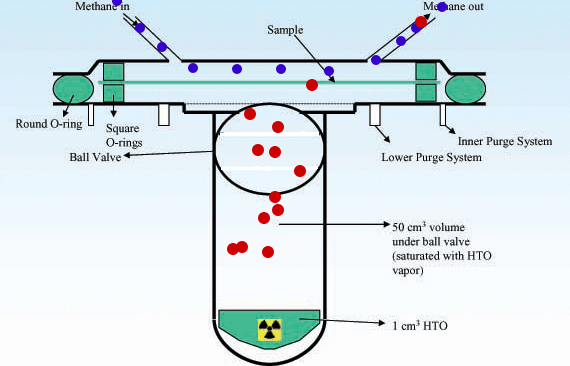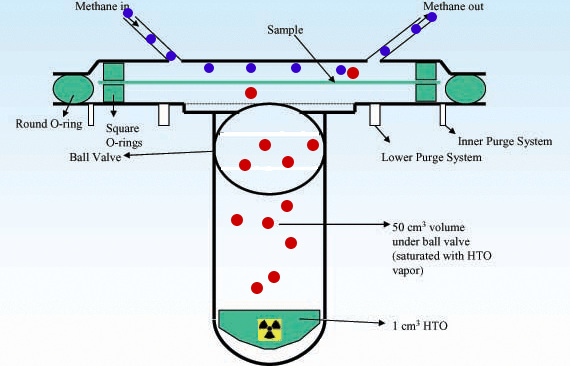 |
Radioactivity Radioactive isotopes such as tritium can be detected after permeation by using counters to detect the products of radioactive decay. Example www.kps.or.kr |
 Techniques for measurement of water vapour sorption and permeation in polymer films, K.A. Schult and D.R. Paul, Journal of Applied Polymer Science 61 (1996) 1865-1876. |
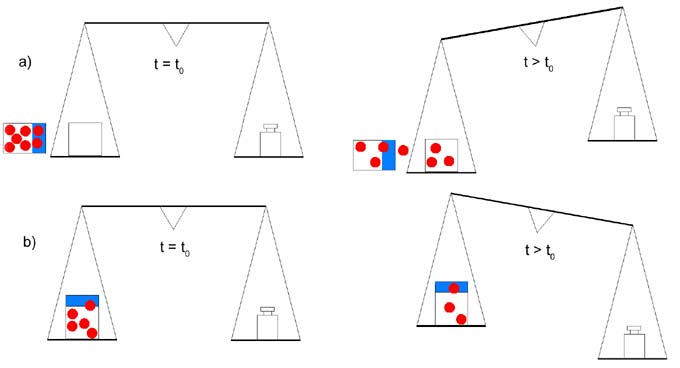
Gravimetric Method (Cup Method) a) "Dry Cup": The permeant is absorbed by an absorbing material such as CaCl2.
The weight increase is measured with a sensitive microbalance. Example www.carlisleccw.com |
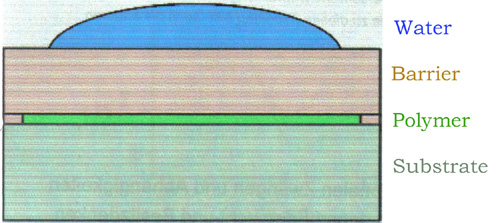 |
X-ray reflectivity Measurements A polymer layer is used to take up the water vapour, which has permeated through a barrier layer. This leads to a change of thickness of the polymer, which can be detected by X-ray reflectivity measurements under very small angles of incidence. Example NIST-website |
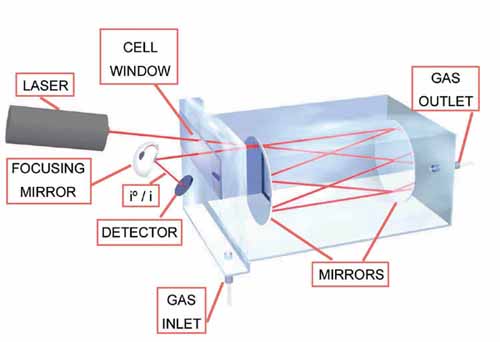 |
Spectroscopic Methods Tunable Diode Laser Absorption Spectroscopy (TDLAS) and Cavity Ring Down Spectroscopy (CRDS) use the absorption of laserlight to estimate the concentration of certain species in the gas cell. Example TDLAS Example CRDS Other optical Methods |
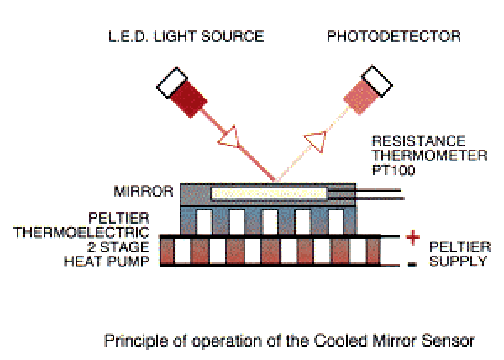 |
Dew point The amount of moisture in a volume can be determined with the dew point method by cooling a mirror. The dew point, where condensation starts on the mirror, can be detected by reflectivity measurements. Tables give the concentration of water in dependence on the dew point. Example www.michell.co.uk |
|
Surface Methods There are a number of methods, which use change of a physical property after adsorption on a surface. Examples are: Quartz Crystal Oscillator, Atom Probe Techniques and Surface Acoustic Wave Methods. |
|
These two tools allow to simulate the Diffusion |